Every surface has energy.
You wouldn’t necessarily think so, but they do. You can’t ‘see’ surface energy just by looking at a material, and neither can you feel or otherwise detect different energies across surfaces by touching them. In everyday life, surface energy isn’t particularly important – but when it comes to adhesive bonding, it most certainly is.
Have you ever noticed how some materials are harder than others to bond with adhesives? Plastics such as polyethylene (PE) and polypropylene (PP) are two good examples of these. These plastics are ubiquitous, found everywhere from plastic bags and bottles to car parts and medical devices. However, despite being all around us, they’re difficult to bond because they have a low surface energy. In this article we’ll explore what this means, why it matters and what you can do to overcome this challenge.
Most common low surface energy (LSE) plastics:
- Polypropylene (found in food packaging, car parts & household goods)
- Polyethylene (used for food packaging, in textiles and medical devices)
- PET (commonly used in plastic bottles and cleaning product containers)
- PTFE (also known as Teflon®, most commonly used in non-stick pans & cookware)
- TPO (Thermoplastic Polyolefins) – used in toys, packaging and pipes
What is surface energy, and why does it matter for adhesives?
Surface energy describes the ‘interaction potential’ of molecules present on a material’s surface. When a material is in bulk form (think deep inside a rock or piece of wood) molecules are surrounded and bonded on all sides. To create a surface, some of these molecular bonds have to be broken, and are therefore no longer fully surrounded by other molecules. The process of surface creation thus creates potential for these newly broken bonds on the surface to form bonds with other molecules. High surface energy materials have molecules that are strongly attracted to molecules from other materials, such as liquids, and readily form new bonds with these molecules. Low surface energy materials are the opposite – their broken bonds aren’t so attracted to other molecules and therefore don’t form bonds easily with other materials.
A surface’s energy can be roughly estimated by putting water onto it. Low surface energy materials cause liquids to bead up – if a surface with zero surface energy existed, water would form a perfect sphere on it. Whereas on a high surface energy material, the water spreads out evenly across the surface. We call this ‘wettability.’ For an adhesive to properly bond with a surface, a higher wettability is preferred as it allows the adhesive to fully spread out and key into the material being bonded.
You may also hear surface energy described in terms of polarity. Polar surfaces have many atoms with a partial charge, such as oxygen, nitrogen or phosphorus atoms. Polar surfaces interact well with liquids and have good wettability. Non-polar surfaces do not have such atoms in substantial enough quantities to enable good wettability. Silicone, Teflon®, polyethylene (PE) and polypropylene (PP) are good examples of non-polar surfaces.
Measuring surface energy
Surface energy is measured in dynes, and an effective way of measuring a material’s surface energy is by using dyne pens. These pens contain a liquid ink with a set surface tension, known as a dyne level. This ink is then applied onto the surface to be bonded to test its surface energy. If the ink forms a continuous film and stays like this for at least 3 seconds, the surface energy is equal to or higher than the pen’s dyne value. Conversely, if the ink bunches up, the surface energy value is lower than that of the pen. A pen with a lower dyne level can then be used to try to work out the material’s surface energy.
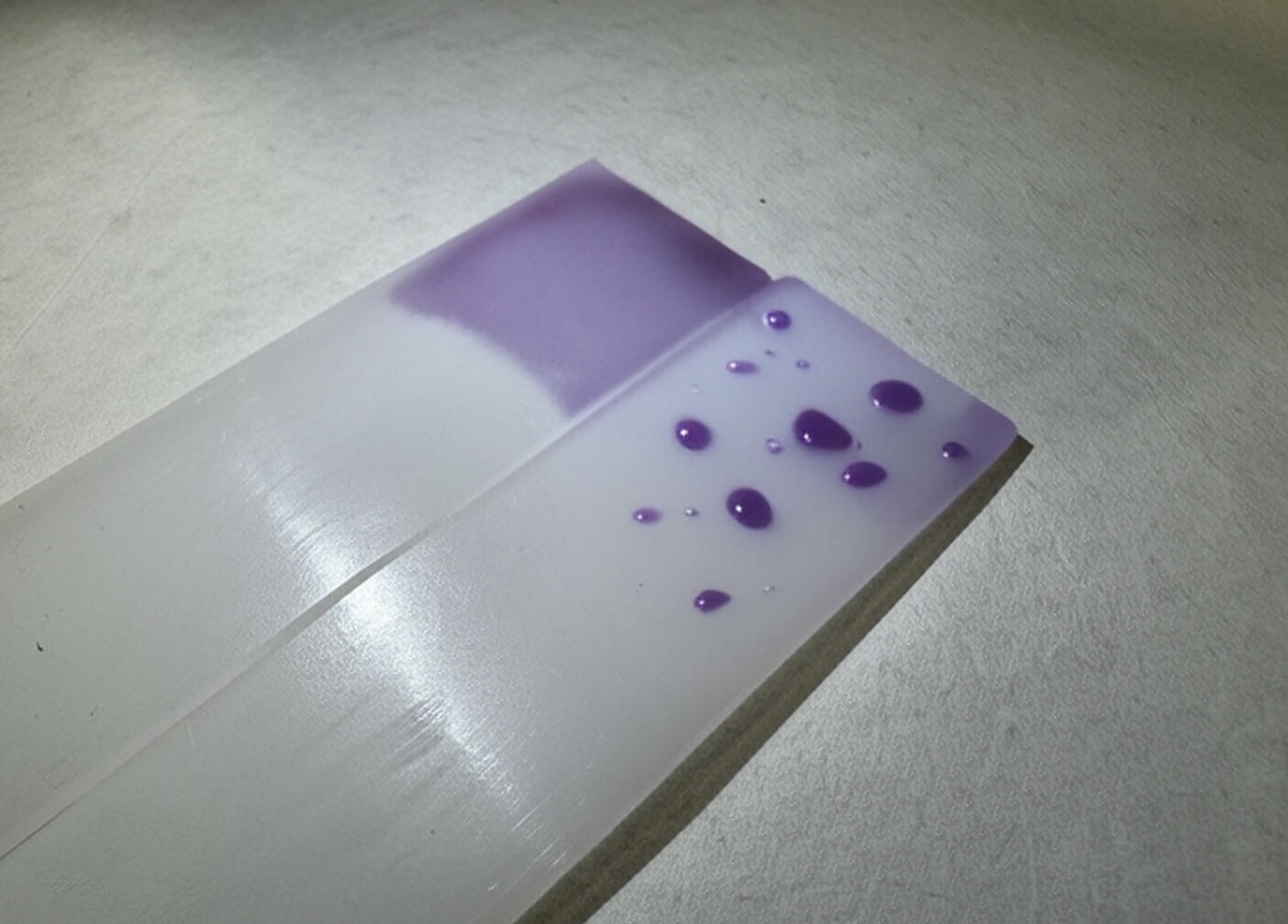
Can surface energy be altered?
Let’s say you want to bond two pieces of PP together, or PE to Teflon®. Can you somehow change the surface energy of these plastics to enable a better adhesive bond? In short – yes, you can. We refer to this as surface treatment or surface pre-treatment, and there are several ways you can do this. However, many of the methods are either expensive and/or time consuming to carry out. Let’s explore some of the most popular methods for increasing surface energy below.
Plasma Treatment
Plasma treatment is a common method for increasing a surface’s wettability. It’s a two-stage process in which a surface is first cleaned with energised, ionised gas (plasma). This helps to remove any volatile organic compounds (VOCs) and other impurities on the surface that could hinder bonding. Afterwards, a layer of plasma containing many polar atoms such as oxygen or nitrogen is directed towards the surface, modifying it and making it more hydrophilic (wettable).
Benefits of plasma treatment include:
- A long-lasting raised surface energy and wettability, depending on the substrate
- Easy to carry out
- An environmentally friendly process
Drawbacks of plasma treatment:
- Initial investment for the equipment can be high
- Proper ventilation is important to ensure outflow of any hazardous gases
- Some substrates don’t stay active for as long as others
- Overtreatment can be as bad as undertreatment, care needed

Corona Treatment
Corona treatment is somewhat similar to plasma treatment in that it modifies a material’s surface, both cleaning it of VOCs and increasing wettability. The difference is that it achieves this via a high voltage electric discharge directly onto the surface.
A high voltage, high frequency generator is connected to an electrode. The high voltage then causes molecules in the air to break down, creating plasma which is then directed at the material’s surface, leading to a similar outcome as with plasma treatment.
Benefits of corona treatment:
- Broadly similar results to plasma treatment
- Good for treating large surfaces in continuous production lines
Downsides of corona treatment:
- Produces ozone gas, which can be harmful
- Generates a lot of static charge which can attract dust and constitutes a safety hazard
- Some materials don’t stay active for long
Flame Treatment
This method of increasing surface wettability involves passing the material to be bonded near to a flame for several seconds. This flame oxidises the surface, creating a thin layer of polar atoms that increase wettability for adhesive bonding.
Benefits of flame treatment include:
- Relatively inexpensive
- Quick to carry out, especially on smaller surface areas
Potential drawbacks of flame treatment include:
- Issues with consistency – it can be challenging to ensure an even spread of flame across an entire surface, especially if it’s large. Therefore, a great deal of precision is required.
- Due to this, higher waste is a possibility due to poorly treated areas of parts
- Fire is a safety risk
- Some emissions are released from this process, not as environmentally friendly as plasma or corona treatment
- Risk of overtreatment / damage to parts
Chemical Etching
Chemical etching involves the application of an acid to a surface in order to oxidise it. Etchants such as Tetra Etch® can be applied to polyolefins such as PE and PTFE to prepare them for adhesive bonding. They do this by reacting chemically with the surface to create a reactive film that forms strong bonds with adhesives. However, chemical etchants are highly hazardous, being corrosive, flammable and toxic. They can only be handled in a fume hood with proper PPE, and due to this, they are often only used in a lab setting.
Permabond TA46XX – the solution for bonding low surface energy plastics
The Permabond TA46XX series of structural acrylic adhesives was developed specifically for bonding LSE plastics such as PE, PP and even PTFE without the need for any surface treatment beforehand. This means you can now effectively bond these surfaces as they are, with only a wipe with isopropanol or another cleaning substance needed before bonding. Using TA46XX is therefore cheaper, quicker and safer than any of the surface treatment methods listed above, and the bonds achieved are no less effective.
Adhesives like Permabond TA4610 and TA4611 provide strong, long-lasting bonds on LSE plastics, as well as on a host of other materials like metals and composites. They cure fully at room temperature and offer excellent chemical, impact and stress resistance. Low-odour variations such as Permabond TA4631 offer similar benefits with increased worker comfort.
You can find a lot more information on TA46XX on our website, for example here and here. If you’d like to request a product sample or discuss your application with us, please get in touch.
Contáctenos
Productos
Encontrar un distribuidor

Los productos Permabond se comercializan a través de distribuidores en todo el mundo.
Certificado ISO
Permabond es una compañía que cuenta con la certificación ISO QMS Certified.